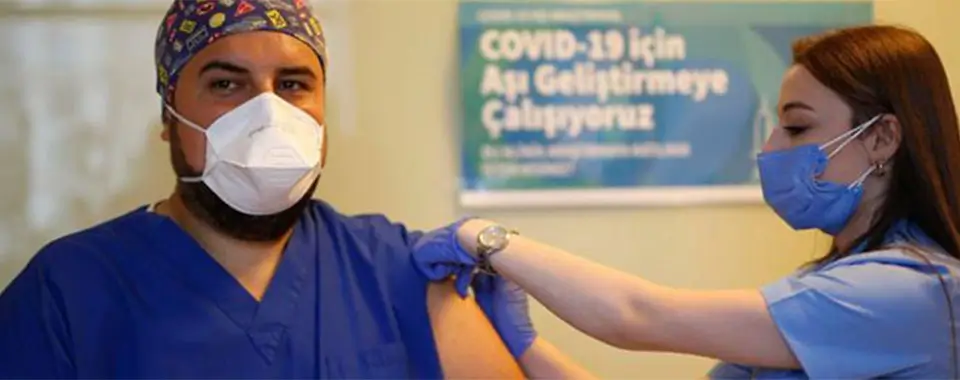
As the world races to find a vaccine for COVID-19, our colleague Dr Cem Gun, an Emergency Medicine Physician at Acıbadem University Atakent Hospital, has bravely volunteered for the country’s first clinical trial. On 9 October, he was administered the first dose of a COVID-19 vaccine developed in China and approved for trial by the Health Institutes of Turkey, at a high-key milestone event.
On why he volunteered for this trial, Dr Cem Gun said, “As a healthcare worker, I fall into the high-risk group for COVID-19 infection. I decided to be vaccinated not only to protect myself but also my patients and loved ones. I made this decision with peace of mind as it is a controlled trial; it is currently in Phase 3, making it a tested and safe vaccine.”
The vaccine will be administered in two doses, and volunteers will be monitored on its effectiveness over a year.
The World Bank has approved US$12 billion in financing to help developing countries buy and distribute COVID-19 vaccines, tests and treatments, aiming to support the vaccination of up to 1 billion people.